A single unit of electro-chemical generator is known as an Electrical Cell and a combination of several such units or electric cells that are electrically connected is called as a battery.
A battery is formed by combining several cells and connecting them electrically in series or parallel to form a battery with two main terminal electrodes, one positive and one negative.
Cathodes are elements with the highest negative electrode potential, while anodes are elements with the highest positive electrode potential. The terminal voltage is provided by the difference between the electrodes. The electrical potential difference between the two main electrodes is determined by the number of cells, cell type, and combination used to create the battery.
The battery generates electrical energy through an electrochemical reaction between two metals with different affinities. When metals are exposed to acids, a voltage develops between them as a result of ion transfer, and closing the circuit induces a current.
An electrical battery is represented by the following symbol in electronic circuits and diagrams: 
A battery is rated in ampere-hours (Ah). This specifies how much charge a pack can hold.
Batteries are made to order for a specific use, and manufacturers are well aware of consumer demands. The mobile phone and electric vehicle markets are two instances of creative adaptations at opposing ends of the spectrum. Unlike consumer batteries, which prioritise small size, high specific energy, and low cost, industrial batteries prioritise consistent performance and long life. The importance of safety in all applications cannot be overstated.
Battery in Series connection:
Higher operating voltages can be obtained by connecting the positive terminal of one cell to the negative terminal of another. When we connect our batteries in series, we double the voltage while keeping the same capacity rating (amp hours). This could be used in a scooter, a Power Wheels vehicle for kids, or other applications. We could simply connect the negative of the first battery to the positive of the second battery with a jumper wire.
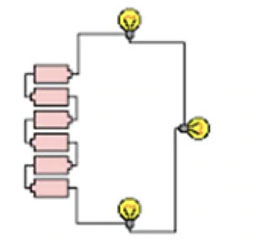
Battery in Parallel connection:
In parallel connection, although the capacity will be increased, the power supply will be provided by one of the individual batteries. To put it another way, when batteries are connected in parallel, the voltage remains constant while the power (or available current) increases. As a result, the batteries will last longer. In other words, the two batteries could power a 6 Volt item for twice as long as a single battery could.
To connect two batteries in parallel, use a jumper wire to connect both positive terminals and another jumper wire to connect both negative terminals of both batteries.

Batteries are classified according to three factors: chemistry, voltage, and specific energy (capacity). A starter battery also produces cold cranking amps (CCA), which refers to its capacity to provide high current at low temperatures.
Chemistry:
Lead, nickel, and lithium are the most popular battery chemistries, and each system requires its own charger. Charging a battery with a charger made for a different chemistry may appear to operate at first, but the charge may not be properly terminated. When transporting and disposing of batteries, keep in mind that each chemistry has its own set of regulations.
Voltage:
Although batteries are labelled with their nominal voltage, the open circuit voltage (OCV) on a fully charged battery is 5–7% higher. The OCV is determined by chemistry and the number of cells connected in series. The operating voltage is the closed circuit voltage (CCV). Before connecting a battery, always check for the correct nominal voltage.
Capacity:
Capacity represents specific energy in ampere-hours (Ah). The discharge current that a battery can provide over time is measured in Ah. You can install a battery with a higher Ah than specified for a longer runtime; alternatively, you can use a slightly smaller pack for a shorter runtime. Chargers are tolerant of Ah rating differences (with the same voltage and chemistry); a larger battery will simply take longer to charge than a smaller pack, but the Ah difference should not exceed 25%.
Types of Battery:
Based on the electric properties batteries can be classified into two main groups:
Primary Battery:
Primary battery is the type of battery which is made up of primary cells (In a primary cell chemical reaction is irreversible). The energy is inherently present in the cells of Primary Battery. These kind of batteries are non-rechargeable and are for single time use. Batteries used in hearing aids (zinc air type) are primary. All AAA, AA, and A type in torches, remote etc are also primary batteries. Examples of primary battery are: Leclanche battery, zinc-chlorine battery, alkaline-maganese battery, metal air battery etc.
Secondary Battery:
Secondary batteries, often known as 'rechargeable batteries', are widely used everywhere. We use them in our house inverter, mobile phones, automobiles and trucks, and rechargeable flashlights.
A secondary battery is built up of secondary cells. Prior to use, energy is induced in the chemistry of the secondary battery's cells by applying external energy or sources. Chemical reactions in secondary cells are reversible.









